Second Law of Thermodynamics
Second Law of Thermodynamics: Overview
This topic covers concepts, such as, Second Law of Thermodynamics, Scope of Second Law of Thermodynamics, Carnot Cycle & Idea behind Carnot Cycle etc.
Important Questions on Second Law of Thermodynamics
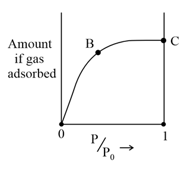
The adsorption of a gas at the boiling point of the gas follows the isotherm shown in the figure. Identify the correct thermodynamic properties at point
A heat engine absorbs heat at temperature and heat at temperature . Work done by the engine is . This data
The unit of is
What does entropy measure?
The change in entropy at equilibrium is
Which of the following statement is NOT correct, according to second law of thermodynamics?
Calculate the increase in entropy (approximate) of three moles of hydrogen gas as it changes from at to and
A Carnot's cycle operates between a source temperature of and a sink temperature of . The engine produces a mechanical work output of per cycle. The heat supplied to the engine by the reservoir is:
Calculate efficiency of a Carnot engine that has its heat souce at and the heat sink at .
Which of the following options is a correct statement regarding a carnot engine?
Enthalpy (H) for the reaction, and emf of the cell was of the cell is?
In Carnot cycle, an ideal gas is taken through 4 reversible steps as shown in the diagram.
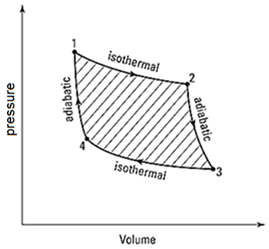
Which of the following statement(s) correct?
(i) ΔU is positive in the step between points 2 and 3.
(ii) ΔU is positive in the step between points 4 and 1.
(iii) ΔU is negative in the step between points 2 and 3.
(iv) Temperature at point 4 is higher than point 2.
What is the temperature T if Carnot’s cycle is said to have efficiency when it operates between T (source) and (sink)?
Calculate the work done by engine if there are no frictional losses and engine operating between and takes heat from a higher temperature reservoir.
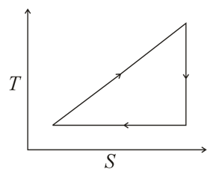
What is the efficiency of a cycle when a working substance goes through the cycle within which the absolute temperature varies fold and the shape of the cycle is shown in the figure ( is the absolute temperature and the entropy)?
Find the amount of heat energy supplied to a Carnot engine from the source in each cycle if the engine is working between and and it has a work output of per cycle.
At a certain temperature efficiency of a Carnot's reversible engine is . When the temperature of the sink is reduced by 62 K, the efficiency of Carnot's cycle becomes double. The temperature of the source and the sink, in kelvin, are respectively --
Find the amount of heat in kcal rejected to sink when an ideal gas heat engine operates in a carnot cycle between 227oC and 127oC and absorbs 6 kcal of heat at higher temperature.
A heat engine based on carnot cycle converts one fourth of heat input into work. When the temperature of the sink is reduced by its efficiency is doubled. The temperature of the source is
Find the temperature of sink for a Carnot's engine which takes calories of heat at and rejects calories of heat to the sink.
